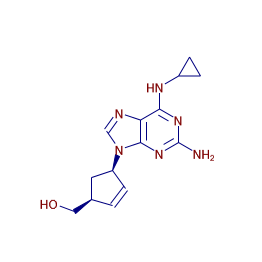
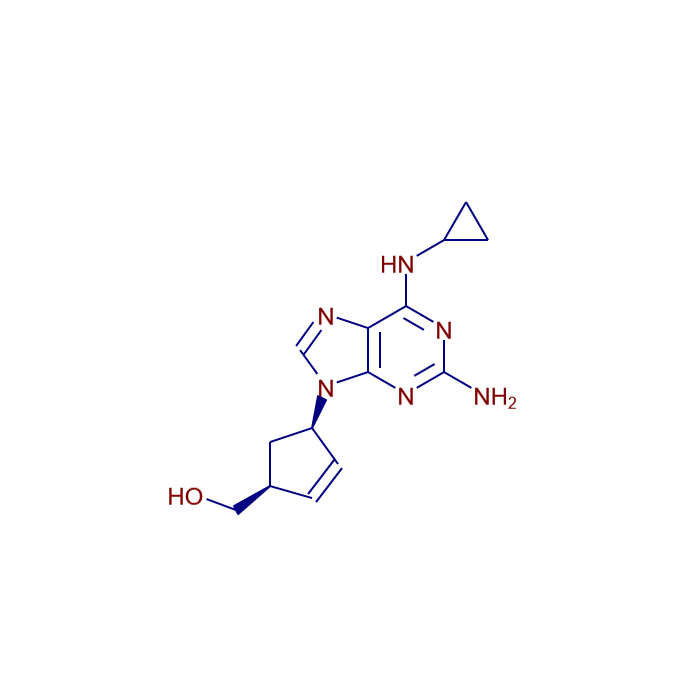
Abacavir
Systematic / IUPAC Name: {(1S,4R)-4-[2-Amino-6-(cyclopropylamino)-9H-purin-9-yl]-2-cyclopenten-1-yl}methanol
ID: Reference7548
Other Names:
Epzicom;
(1S,4R)-4-[2-Amino-6-(cyclopropylamino)-9H-purin-9-yl]-2-cyclopentene-1-methanol;
{(1S,4R)-4-[2-Amino-6-(cyclopropylamino)-9H-purin-9-yl]cyclopent-2-en-1-yl}methanol ;
{(1S,4R)-4-[2-Amino-6-(cyclopropylamino)-9-purinyl]-1-cyclopent-2-enyl}methanol ;
{(1S,4R)-4-[2-Amino-6-(cyclopropylamino)purin-9-yl]-1-cyclopent-2-enyl}methanol
; more
Formula: C14H18N6O
Class: Therapeutics/Prescription Drugs
Spectral Data
Abacavir mass spectral data can be found in a separate interface. The data are manually curated and of the highest quality.
Available MS Data
| Used Instruments | Q Exactive Orbitrap |
| No. of Spectral Trees | 1 |
| No. of Spectra | 345 |
| Tandem Spectra | MS1, MS2 |
| Ionization Methods | ESI |
| Analyzers | FT |
| Last Modification | 3/15/2018 2:19:16 PM |
Identificators
| InChI | InChI=1S/C14H18N6O/c15-14-18-12(17-9-2-3-9)11-13(19-14)20(7-16-11)10-4-1-8(5-10)6-21/h1,4,7-10,21H,2-3,5-6H2,(H3,15,17,18,19)/t8-,10+/m1/s1 |
| InChI Key | MCGSCOLBFJQGHM-SCZZXKLOSA-N |
| Canonical SMILES | C1CC1NC2=NC(=NC3=C2N=CN3C4CC(C=C4)CO)N |
| CAS | |
| Splash | |
| Other Names |
Epzicom; (1S,4R)-4-[2-Amino-6-(cyclopropylamino)-9H-purin-9-yl]-2-cyclopentene-1-methanol; {(1S,4R)-4-[2-Amino-6-(cyclopropylamino)-9H-purin-9-yl]cyclopent-2-en-1-yl}methanol ; {(1S,4R)-4-[2-Amino-6-(cyclopropylamino)-9-purinyl]-1-cyclopent-2-enyl}methanol ; {(1S,4R)-4-[2-Amino-6-(cyclopropylamino)purin-9-yl]-1-cyclopent-2-enyl}methanol ; {(1S,4R)-4-[2-Amino-6-(cyclopropylamino)purin-9-yl]cyclopent-2-en-1-yl}methanol ; {(1S,4R)-4-[2-Azanyl-6-(cyclopropylamino)purin-9-yl]cyclopent-2-en-1-yl}methanol ; {(1S,4R)-4-[2-Amino-6-(cyclopropylamino)-9H-purin-9-yl]cyclopent-2-en-1-yl}methanol; 2-Cyclopentene-1-methanol, 4-[2-amino-6-(cyclopropylamino)-9H-purin-9-yl]-, (1S,4R)- |
In Other Databases
| Wikipedia | Abacavir |
| ChemSpider | 390063 |
| DrugBank | DB01048 |
| PubChem | 441300 |
| HMDb | HMDB15182 |
| ChEBI | CHEBI:421707 |
| KEGG | D07057; C07624 |
| ChEMBL | CHEMBL1380 |